How Do You Read a Flu Test
Algorithm to assist in the interpretation of influenza testing results and clinical controlling during periods when influenza viruses are circulating in the community
Effigy: Algorithm to assist in the interpretation of flu testing results and clinical decision-making during periods when flu viruses are circulating in the community1
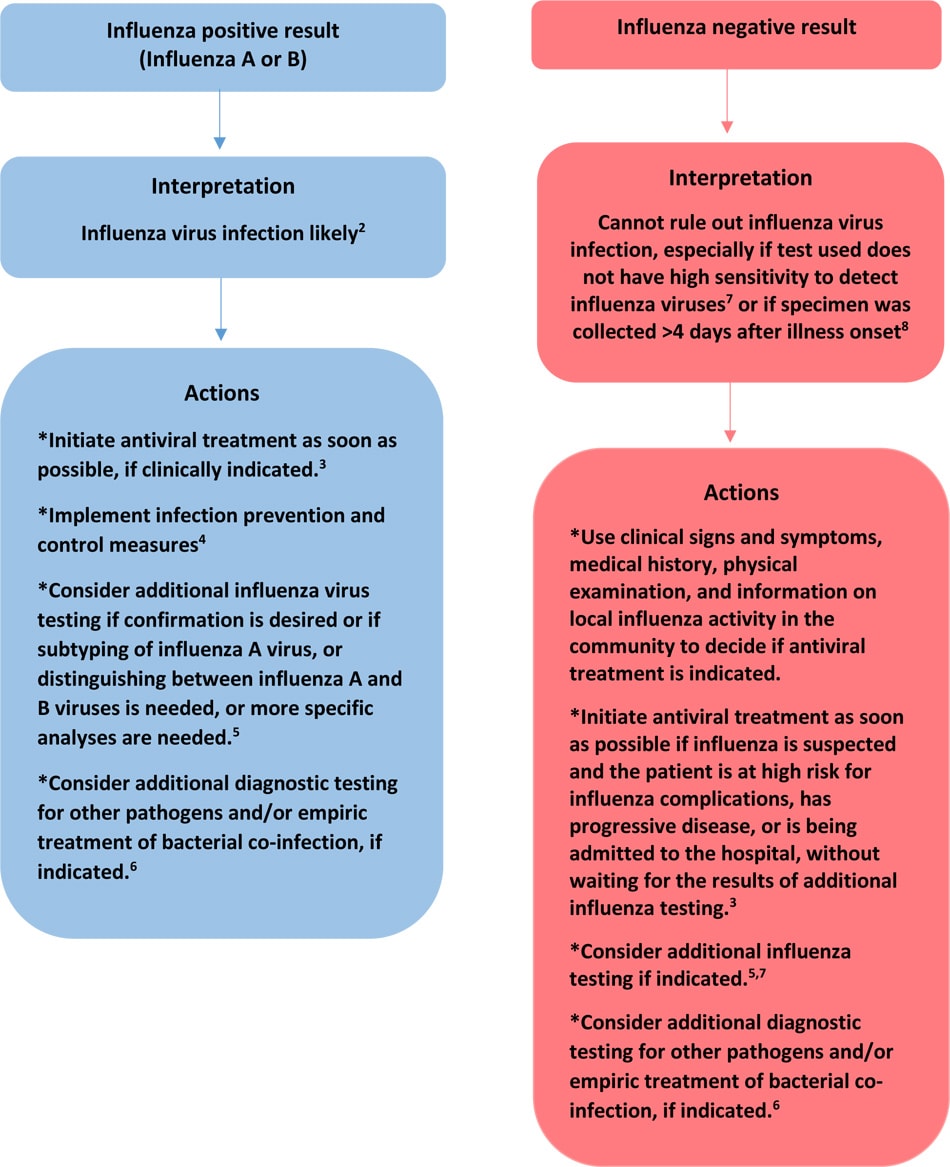
1. During periods when flu activeness is loftier and influenza viruses are circulating among persons in the community, the positive predictive value of a test result is high (that is, the take chances that a positive result indicates that the patient has influenza is high – likely true positive effect), and the negative predictive value of a examination result is low (the chance that a negative event indicates that the patients does not have flu – consider potential for a false negative result) if the flu test used has suboptimal sensitivity (such as a rapid influenza diagnostic test or immunofluorescence assay) to detect influenza virus in respiratory specimens compared to a "gold standard examination" such every bit RT-PCR or viral civilisation.
2. A positive effect of an antigen detection test (rapid influenza diagnostic examination, immunofluorescence analysis) or a molecular assay likely ways that the patient has or has recently had influenza virus infection, but does not always mean that the patient is nevertheless infectious. Only isolation of flu virus by viral culture can identify whether infectious flu virus is nowadays.
3. Antiviral treatment is recommended as soon every bit possible for all of the following who have suspected or confirmed influenza: outpatients who are at loftier-adventure for influenza complications, persons with severe or progressive disease, and hospitalized patients. See Influenza Antiviral Medications: Summary for Clinicians for more information.
four. Come across: Prevention Strategies for Seasonal Influenza in Healthcare Settings
five. Influenza virus infection may include seasonal influenza A(H3N2), A(H1N1)pdm09, influenza B, or rarely, novel influenza A virus infection. The interpretation of positive and negative flu testing results will, in part, depend on the test used, the sensitivity and specificity of the test compared to a "gilt standard test", and the prevalence of influenza in the population being tested. If tests for both influenza A and flu B are positive, refer the respiratory specimen to a public wellness laboratory for resolution, equally influenza A and B virus co-infections are uncommon. Most tests do non distinguish between influenza A virus subtypes, and do not distinguish betwixt seasonal flu A viruses and novel influenza A viruses. If influenza A virus subtyping is needed, specimens should be sent to a state public health laboratory for molecular testing (RT-PCR). If there has been recent exposure to pigs or birds (poultry or wild birds) or to a sick person with such animal exposures and novel influenza A virus infection is suspected, the state wellness department should be notified immediately and respiratory tract specimens should be forwarded for RT-PCR at the state wellness department virology laboratory. Note that recommended infection and prevention control measures should be followed when collecting respiratory specimens, including from patients with suspected novel influenza A virus infection. See Avian Influenza: Data for Health Professionals and Laboratorians and Variant Flu Viruses: Groundwork and CDC Risk Assessment and Reporting for more data.
6. Consult the local or state health department or other sources (due east.yard., virology testing at a local hospital) for local activity on other respiratory pathogens associated with acute respiratory illness. Empiric antibiotic coverage for possible secondary bacterial infections should include coverage for Streptococcus pneumoniae, Staphylococcus aureus (including MRSA), and Grouping A Streptococcus, especially for hospitalized developed patients (see the Infectious Diseases Social club of America (IDSA)/American Thoracic Gild (ATS) Community-acquired Pneumonia guidelinesexternal icon) and for some children (meet the Pediatric Infectious Diseases Gild of America (PIDS)/Infectious Diseases Society of America (IDSA) Community-acquired Pneumonia guidelinesexternal icon).
vii. Antigen detection tests (rapid influenza diagnostic tests, immunofluorescence assays) have suboptimal sensitivities to detect influenza viruses in respiratory specimens compared to molecular assays and viral civilisation and negative results of antigen detection tests should not be used to exclude a diagnosis of influenza. If influenza is suspected, and diagnostic testing is indicated, influenza testing of respiratory specimens by molecular assay (e.k. RT-PCR) should be performed. The Infectious Diseases Society of America (IDSA) recommends use of rapid flu molecular assays over rapid flu diagnostic tests (RIDTs) for detection of influenza viruses in respiratory specimens of outpatients. IDSA recommends use of RT-PCR or other molecular assays for detection of influenza viruses in respiratory specimens of hospitalized patients. Consult the IDSA Influenza Clinical Practice Guidelinesexternal icon for recommendations on influenza testing and data on interpretation of testing results.
eight. Respiratory specimens should be collected as close to illness onset as possible to maximize detection of flu viruses. Influenza viral shedding in the upper respiratory tract declines after almost iii-4 days in well-nigh people. Infants, young children, immunocompromised and immunosuppressed patients tin can shed influenza viruses for longer duration. Molecular assays tin observe flu viral RNA in respiratory specimens for longer periods than antigen detection assays. For hospitalized patients with lower respiratory tract illness and suspected influenza, lower respiratory tract specimens should be collected and tested for flu viruses by RT-PCR because influenza viral shedding in the lower respiratory tract may be detectable for longer periods than in the upper respiratory tract, if flu testing of upper respiratory tract specimens yields a negative result. If the patient is critically sick on invasive mechanical ventilation, and has tested negative on an upper respiratory tract specimen, including past a molecular analysis, a lower respiratory tract specimen (endotracheal aspirate or bronchoalveolar lavage fluid if collected for other diagnostic purposes) should be tested for influenza viruses by RT-PCR or other molecular assays.
Source: https://www.cdc.gov/flu/professionals/diagnosis/algorithm-results-circulating.htm
0 Response to "How Do You Read a Flu Test"
Post a Comment